As part of the Company’s presentation at CPhI Worldwide and AAPS PharmSci360, Quotient Sciences has announced a new service to support clients through earlier stages of drug development.
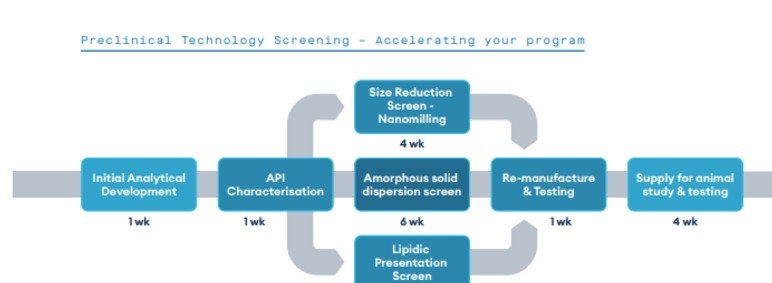
Our new preclinical technology screening service leverages Quotient Sciences’ three-decade history of excellence in solubility enhancement to screen different enabling technologies to improve exposure in preclinical animal models. This helps to, achieve proportional dose-exposure profiles in dose range finding (DRF) studies, and hit targeted high dose toxicology concentrations as per ICHM3R2 guidance.
From our facility in Nottingham, UK, three different platforms can be evaluated in less than 8 weeks, with the generation of fit-for-purpose formulations for dosing in animal non-GLP PK studies over the subsequent 3-4 weeks.
From our facility in Nottingham, UK, three different platforms can be evaluated in less than 8 weeks, with the generation of fit-for-purpose formulations for dosing in animal non-GLP PK studies over the subsequent 3-4 weeks.
In the later stages of a molecule’s development following GLP toxicology studies, we can support a seamless transition into a clinical, Phase I trial with on-demand drug product manufacturing using our Translational Pharmaceutics® platform for integrated drug development.
To learn more about Quotient Sciences’ preclinical toxicology screening service, download our info sheet or contact us to speak about your next program